
Vinay Prasad MD MPH
@VPrasadMDMPH
Prof @ucsf, Physician-Scientist, IG @vprasadmdmph @vkprasadlab @plenary_session, YouTube, #vpzd podcast & @Sensible__Med; Views mine
ID:1194962714
http://www.drvinayprasad.com 18-02-2013 21:24:10
45,1K Tweets
286,7K Followers
3,7K Following




Vinay Prasad MD MPH Worked for a pharma company whose drug had an adverse event signal in a clinical trial in pregnant women.
There were protests in streets and scientific meetings by activists and patient advocacy groups demanding the drug's FDA hold be lifted.
Guess who was funding these groups?

Vinay Prasad MD MPH My concern is that the FDA's Oncology Director explained accelerated approval (AA) in a way that conflicts with the agency's website when he said that AA is about treating serious and life-threatening diseases sooner without consideration for unmet need. Is anyone going to…




Appreciated your commment on toxicity David Mitchell
I think the data that deeper first response means longer life is flawed, and a false story propagated by pharma. regarding your other comment. The poor R2 for MRD and OS proves it is false

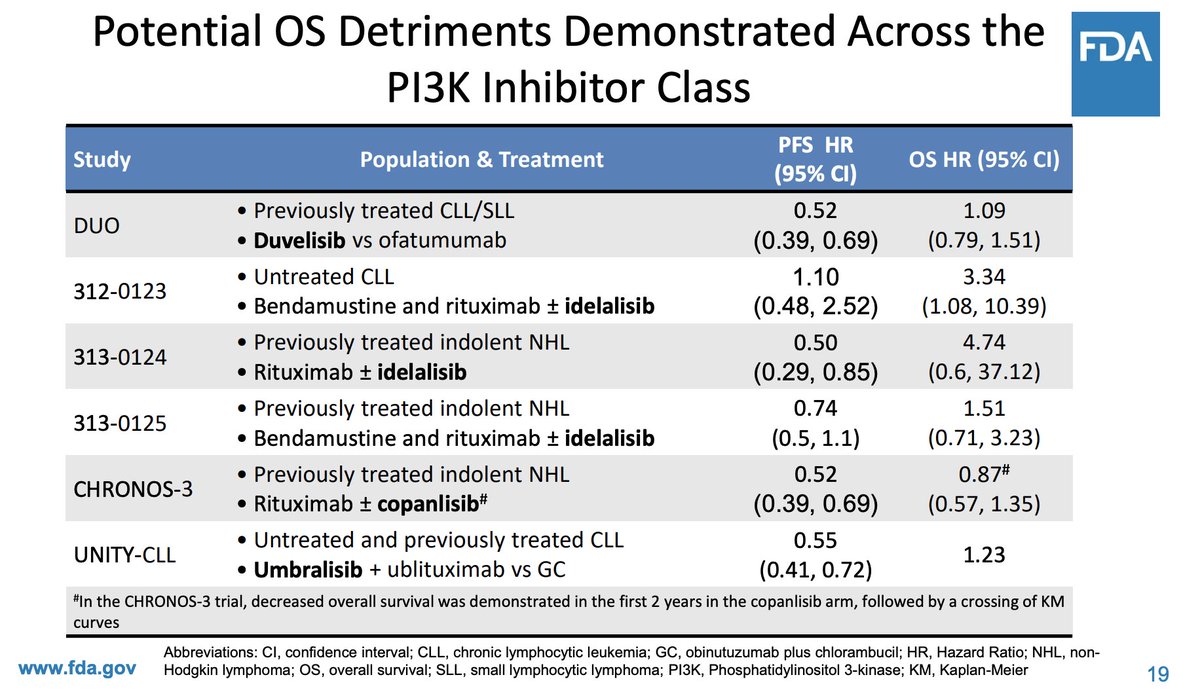
Weird to see FDA discus the P13k failure because their inaction left it on the market for years, as we document in a JAMA Internal Medicine
jamanetwork.com/journals/jamai…


Vinay Prasad MD MPH I also think Pazdur just mispoke about unmet medical need not being part of the regulation.
Per FDA: 'The FDA instituted its Accelerated Approval Program to allow for earlier approval of drugs that treat serious conditions, and fill an unmet medical need based on a surrogate…






Vinay Prasad MD MPH very provocative point of view. With the increased coverage of the rising rates and early detection across types of cancer, not enough discussion on the survival gains or the breadth of the drug class(es) to treat multiple myeloma.


