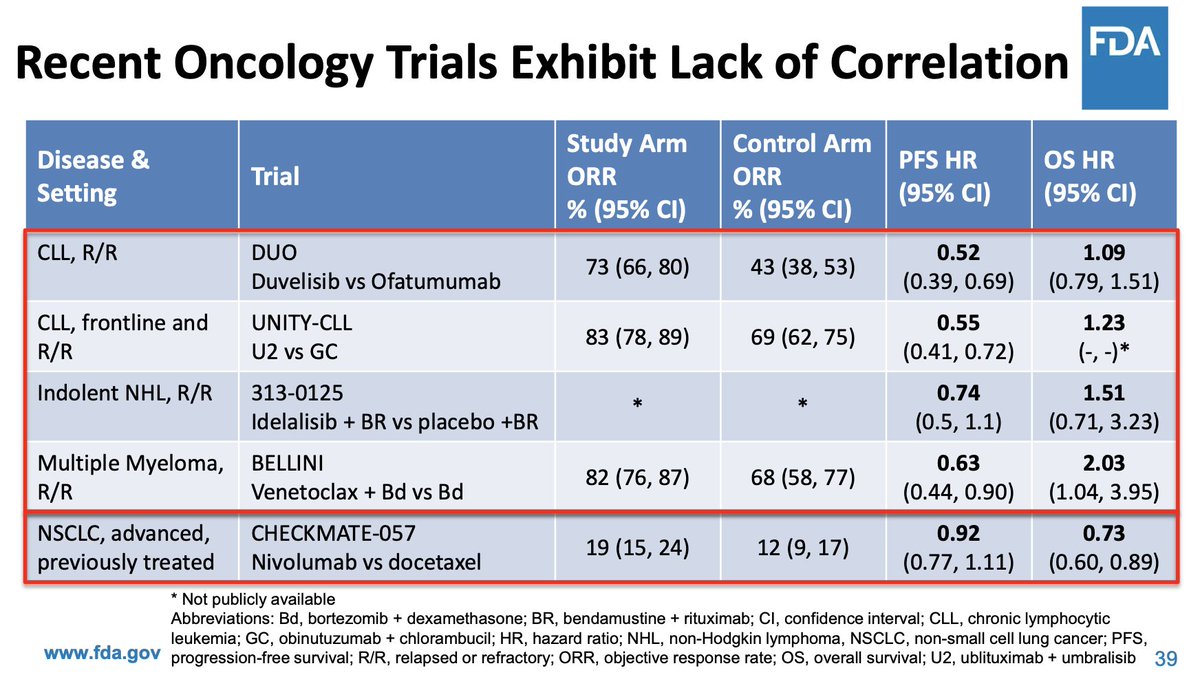
Compared to Comprehensive Genomic Profiling (CGP), single-gene assays are limited to a single biomarker. In contrast, #CGP uncovers many mutations while scanning genomes for multiple biomarkers.
#Genomics #PrecisionMedicine #FDAODAC #ngs #mutations #Cancer #cancerfreeindia



Be a part of the exciting session by Dr. Pramod Kumar Julka to know how #PrecisionOncology is bringing revolution and setting new standards for personalized cancer care.
Register Now: 4basecare.com/poareg/
#Genomics #PrecisionMedicine #CancerEducation #FDAODAC #Illumina #DNA




#FDA schedules Feb 9 advisory panel review for #dostarlimab , submitted by GlaxoSmithKline, for proposed use as monotherapy in patients with mismatch repair deficiency/microsatellite instability-high locally advanced rectal cancer. $GSK #FDA ODAC #adcomm
public-inspection.federalregister.gov/2022-27834.pdf









SITC is pleased to present this report on the April 28, 2021, meeting of the U.S. Food and Drug Administration (FDA) Oncologic Drugs Advisory Committee (ODAC). #FDAODAC #atezolizumab #pembrolizumab
You can view the report on our blog, The Sentinel. blog.sitcancer.org/2021/04/sitc-m…

In April, the Oncologic Drug Advisory Committee (ODAC) voted 7-2 to keep atezolizumab on the market for advanced/metastatic triple-negative breast cancer. National Breast Cancer Coalition was disappointed with this but not surprised. Read our recap. #FDAODAC stopbreastcancer.org/call-to-action…