
Our collective commitment is embodied in the belief that 'Cancer doesn't take a day off: neither do we' - Md Foorquan Hashmi
oncodaily.com/49233.html
#oncodaily #oncology #cancer #FDA #multiplemyeloma #cancer awareness #medicalnews #FDA approval #healthcare

The U.S. FDA recently approved two CAR T-cell therapies for earlier treatment of adults with multiple myeloma - The Leukemia & Lymphoma Society
oncodaily.com/52828.html
#oncodaily #oncology #cancer #CARTcelltherapy #FDAapproval #multiplemyeloma #cancer treatment #LLS #researchmilestones

Great news from Piramal Pharma! The US FDA has given the green light, closing the inspection of their manufacturing facility in Riverview, USA. Big step forward! 🎉 #PiramalPharma #FDAApproval

FDA approves ImmunityBio, Inc.'s Anktiva combined with BCG for BCG-unresponsive NMIBC with CIS, advancing cancer care. #BladderCancerTreatment #FDAApproval #ImmunityBioTherapy
reuters.com/business/healt…

ImmunityBio’s Anktiva got FDA approval!
With almost 38% of the float shorted the SP can run fast and high.
Don’t sell!
#FDAapproval $Ibrx #StockMarket #shortsqueeze #wallstreetbets #ToTheMoon #curecancer


Osimertinib with platinum-based #chemotherapy has received #FDAapproval for patients with locally advanced or metastatic #NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations. ow.ly/rihx50R715M


ARS Pharma has sent a reply to the FDA following a new study on nasal epinephrine. Stay updated! #Healthcare #FDAApproval buff.ly/49JXKnr


BiVictriX Therapeutics receives FDA orphan drug designation for BVX001 in AML business-news-today.com/bivictrix-ther… #BiVictriXTherapeutics #FDAOrphanDrug #AMLtreatment #Biotechnology #FDAApproval #CancerResearch #Leukaemia

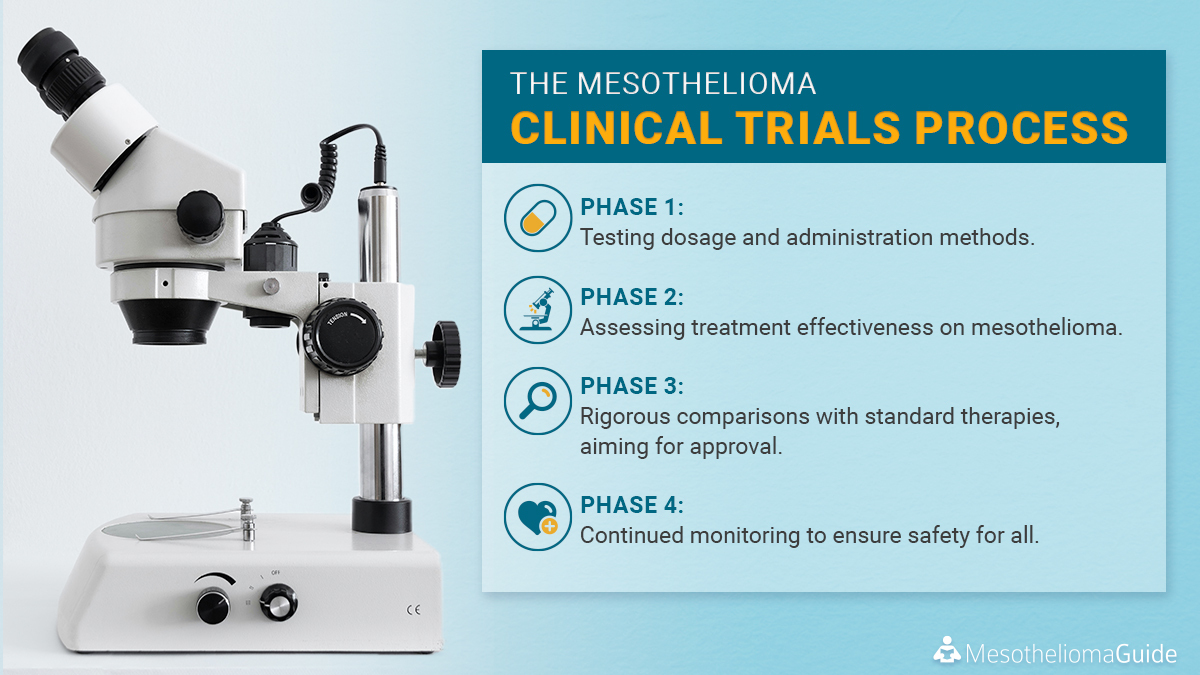
Discover the phases of #mesothelioma clinical trials:
1️⃣ Dosage & administration testing.
2️⃣ Assessing treatment effectiveness.
3️⃣ Comparing with standard therapies for #FDAapproval .
4️⃣ Ensuring long-term safety post-approval.
Read more: tinyurl.com/ynt35k8x

FDA approves Lumisight for breast cancer lumpectomy procedures, aiding surgeons in identifying cancerous tissue. Clinical trials show effectiveness in reducing the need for additional surgeries. #CancerDetection #Lumisight #FDAApproval
fda.gov/drugs/news-eve…


🎉 Exciting news alert! 🎉 The FDA has just expanded the labels of two CAR T-cell products allowing for the use of these innovative treatments in earlier lines of treatment. 🙌 #FDAapproval #multiplemyeloma #CAR -Tcelltherapy #innovation #fightingcancer
hubs.ly/Q02sLtRP0

Osimertinib with platinum-based #chemotherapy has received #FDAapproval for patients with locally advanced or metastatic #NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations. ow.ly/rihx50R715M


The FDA has approved ImmunityBio's Anktiva, an IL-15 receptor agonist immunotherapy, for patients with Bacillus Calmette-Guérin (BCG) unresponsive non-muscle invasive bladder cancer (NMIBC).
U.S. FDA ImmunityBio, Inc. #FDA #FDA approval #ImmunityBio #pharmanews
pharmamanufacturing.com/development/dr…
🎉👏 FDA gives green light to Culver City company's bladder cancer treatment! 🙌🔬 Stay updated on the latest breakthrough in cancer research. #FDAApproval #BladderCancerTreatment #MedicalAdvancements
latimes.com/business/story….

$IBRX 🚀 FDA approves ANKTIVA, a first-in-class IL-15 receptor agonist for BCG-unresponsive Non-Muscle Invasive Bladder Cancer. With strong data on durable responses and minimal side effects, ANKTIVA is poised to revolutionize cancer treatment. #ImmunityBio #FDAApproval

Lilly announces significant results in SURMOUNT-OSA trials of tirzepatide in OSA business-news-today.com/lilly-announce… #Lilly #SURMOUNTOsa #ClinicalTrials #SleepApnea #HealthcareInnovation #FDAApproval

Kim Moran, SVP, head of U.S. Rare Diseases at UCB, discusses Zilbrysq and the Phase 3 study that led to its approval #checkrare #FDAApproval #MyastheniaGravis
checkrare.com/the-data-that-…
