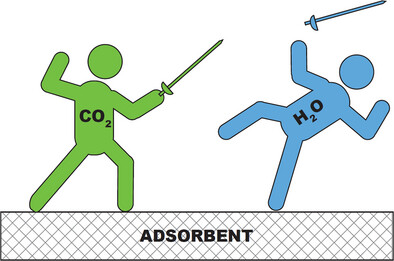
Tailoring Hydrophobicity and Pore Environment in Physisorbents for Improved Carbon Dioxide Capture under High Humidity
J. Am. Chem. Soc. #Chemistry #Science
pubs.acs.org/doi/10.1021/ja…






#CrystalEngineering |s of Two Light and Pressure Responsive Physisorbents (Michael J. Zaworotko and co-workers) Mike Zaworotko group #openaccess onlinelibrary.wiley.com/doi/10.1002/an…
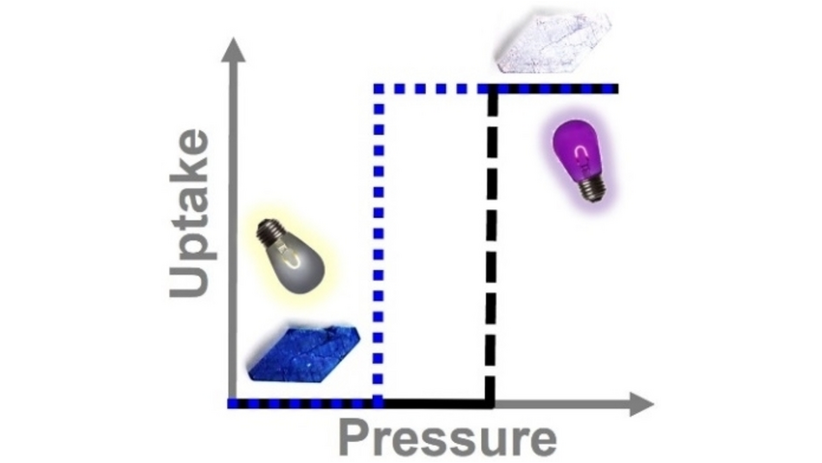
When 'too good to be true' means what it says
@ZgroupUL has had many 'too good to be true' events over 37 yrs.🇮🇪🇨🇦🇺🇸 Some, (ionic liquids, ultramicroporous physisorbents, pharmaceutical cocrystals) are the real deal + general in nature. Most were not.
cendigitalmagazine.acs.org/2022/02/14/met…

Tailoring Hydrophobicity and Pore Environment in Physisorbents for Improved Carbon Dioxide Capture under High Humidity | JACS Randall Snurr The Farha Group MOFia Northwestern Chemistry Northwestern #Hydrophobicity #Pore #Physisorbents #CO2 #Capture pubs.acs.org/doi/10.1021/ja…

Theoretical chemists Theoretical Chemistry Group (Chuo Univ., Tokyo) and chemical engineers at Nihon Univ. collaborated with Kanazawa Univ. & RITE to establish an optimization protocol of CO2 physisorbents based on electronic structure informatics & precision measurements.
go.acs.org/4Io

David Paul Iacomi @lscottblank Marco Taddei ....main issue with amines can be deactivation in the presence of oxygen. NbOFFIVE-1-Ni and SGU-29 are the leaders here for physisorbents. SGU-29 edging it as it has specific binding sites for CO2 and H2O, which overcomes coadsorption issues. SIFSIX-18-Ni good too Soumya Mukherjee

David Paul Iacomi @lscottblank Isotherms for amine modified materials have very steep uptake at low pressures. The kinetics at low partial pressures however is a bit slower than physisorbents but as Marco Taddei mentioned water has little effect and can be beneficial....

David Paul Iacomi @lscottblank To the best of my knowledge, there are only a handful of physisorbents that can capture appreciable amounts of CO2 at 400 ppm: SIFSIX-3-Cu, NbOFFIVE-1-Ni (both MOFs) and SGU-29 (a copper silicate). They have Qst in the region of 50-60 kJ/mol, so are rather strong physisorbents.