Zydus receives final approval from USFDA for Tretinoin Cream USP, 0.1% ✅
#zyduslife #sabarisecurities




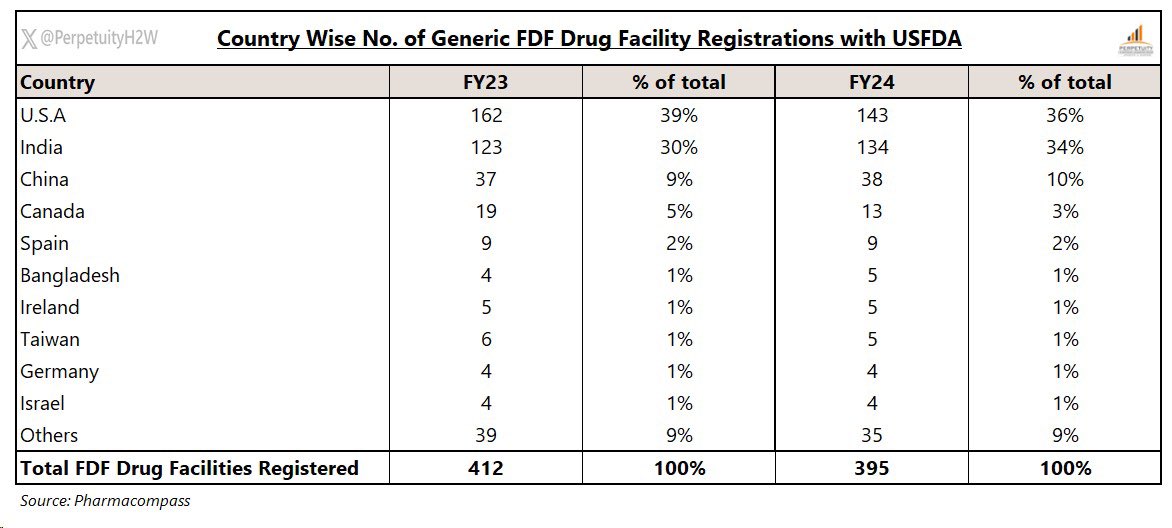
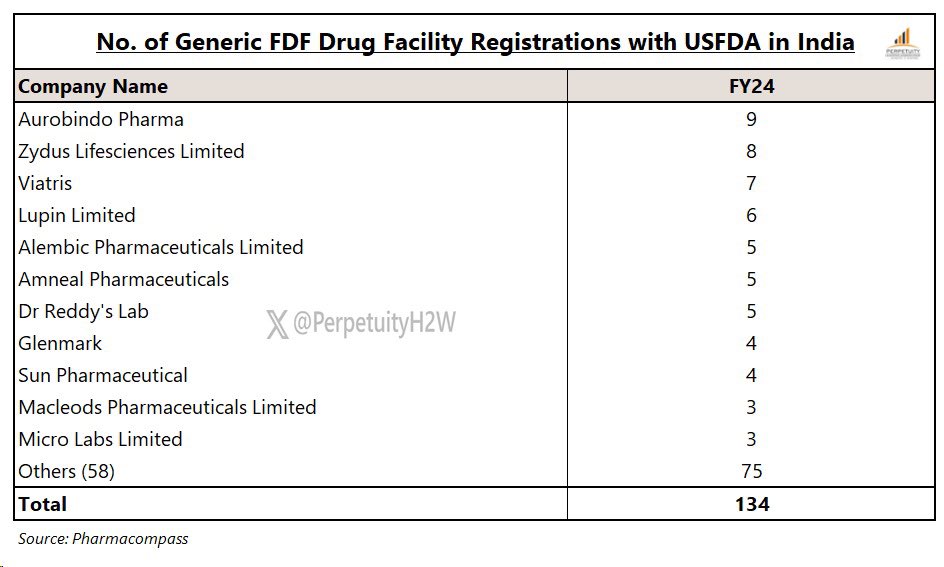
India had 134 #FDF registered facilities with #USFDA in FY24
11 co.s accounted for ~44% of USFDA registered formulation facilities in India.
Highest no. of FDF USFDA registered facilities were with #Aurobindo (9) followed by #Zydus (8)
#Health2Wealth #Perpetuity

By pinpointing muscle dysfunction early on, you can tailor your treatment plan more effectively and potentially alleviate your low back pain.
#BackPain #MuscleHealth #EMG #StayStrong #startoonlabs #pheezee #iso13485 #iso9001 #FDAcleared #USFDA #patent #patent ed #medicaldevice



The U.S. FDA has determined that the inspection classification of the facility is Voluntary Action Indicated (VAI), Lupin said.
#Lupin #USFDA #inspection #pharma #health #health care #rupee #news #marketupdates #marketupdates #stockinfocus #StocksToBuy #federal #bseindia …


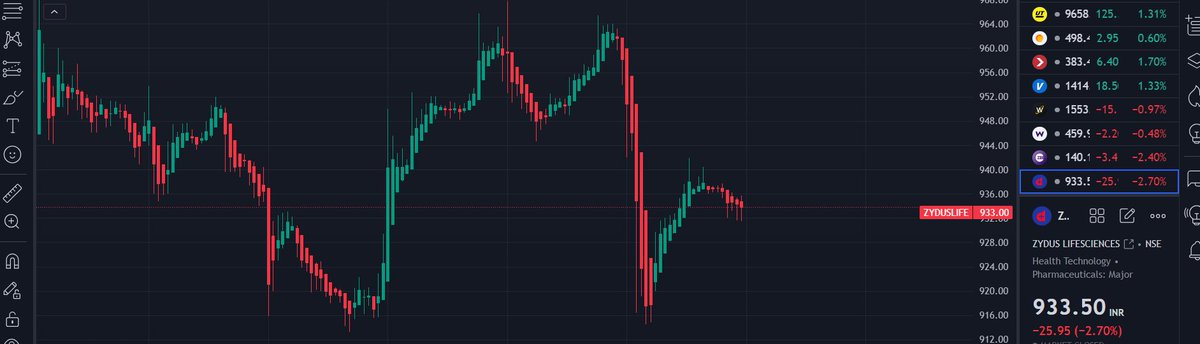
Zydus Lifesciences reports that the USFDA inspection at their facility near Vadodara, in Jarod, has concluded with 10 observations.
#Nifty #Nifty 50 #banknifty #niftybank #Dow #SP500 #Nasdaq #stockmarket #Bitcoin #zerodha #stocks



Glenmark Pharma Receives USFDA Approval for Generic Anti-Inflammatory Drug
barawakar.com/glenmark-pharm…
#GlenmarkPharma
#USFDAApproval
#GenericDrugs
#AntiInflammatory
#Healthcare
Discover how Glenmark Pharmaceuticals is revolutionizing the healthcare landscape with its latest USFDA


Zydus Lifesciences’ Injectable Manufacturing Site Successfully Concludes USFDA Inspection
barawakar.com/zydus-lifescie…
#ZydusLifesciences
#USFDAinspection
#PharmaceuticalQuality
#ComplianceExcellence
#InjectableManufacturing
Discover the latest developments as Zydus Lifesciences



Flaccidity marks the beginning of the hemiplegia journey. Leveraging with pheezee we can intervene early to pave the way for recovery
#EarlyIntervention #Hemiplegia #Awareness #SEMG #ROM #startoonlabs #pheezee #iso13485 #iso9001 #FDAcleared #USFDA #patent #patent ed #medicaldevice