Sandy Wong
@SandyWong02111
ID:4050824739
27-10-2015 04:10:49
4,1K Tweets
1,4K Followers
160 Following


Published today in Blood Journal 🎉 Efficacy of CAR T-Cell Therapy is Not Impaired by Previous Bispecific Antibody Treatment in Large B-Cell Lymphoma #CART #lymsm
Great collaboration with French colleagues Roch Houot Lysa Lymphoma Pere Barba
pubmed.ncbi.nlm.nih.gov/38657242/


International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of TCE bispecific antibodies in MM #mmsm The Lancet Oncology Paula Rodriguez-Otero Saad Z. Usmani MD MBA FACP 🇺🇸🇵🇰 Jonathan Kaufman, MD Rakesh Popat thelancet.com/journals/lanon…

Cinco de Myeloma (observed)! ! Love celebrating with my UCSF Helen Diller Family Comprehensive Cancer Ctr family! Sandy Wong Ajai Chari Tom Martin JEFFREY WOLF Alfred Chung



Real world data on bridging therapy for CAR-T. Report by U.S. Myeloma Immunotherapy Consortium Aimaz Afrough Yi Lin Surbhi Sidana, MD Gurbakhash Kaur Larry Anderson,MD,PhD,FACP Shonali Midha, M.D. Hamza Hashmi Krina Patel Douglas W. Sborov MD MS Omar Nadeem et al.
nature.com/articles/s4140…

CONGRESS | #EBMT24 | POSTER | Reuben Benjamin King's College London shares the final analysis of the LocoMMotion study in relapsed/refractory #multiplemyeloma .
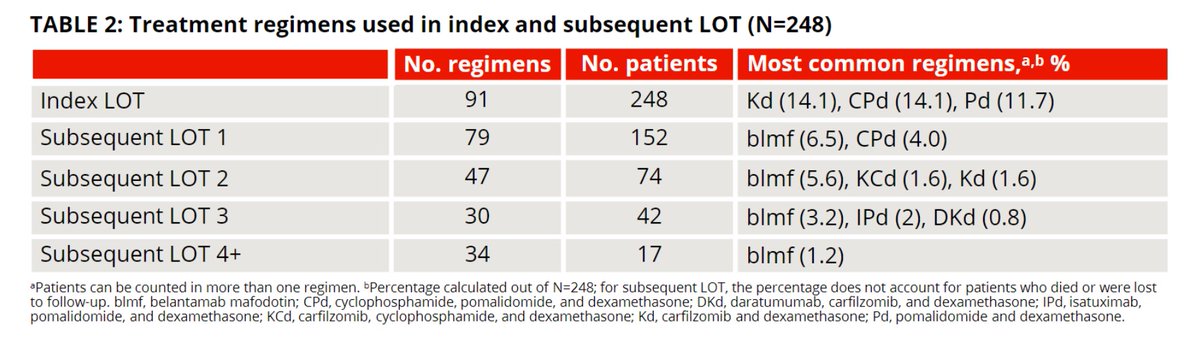
24-mo PFS 10.5%, 24-mo OS 33.7%, pts treated with a subsequent LOT 61.3%, 79 different regimens were used in subsequent LOT1,



BCMA- or GPRC5D-targeting bispecific antibodies in multiple myeloma: efficacy, safety, and resistance mechanisms #mmsm #bmtsm nizar jacques bahlis Blood Journal sciencedirect.com/science/articl…